Material properties
Questalpha GmbH & Co. KG manufactures a cellulose sponge material effectively used in various medical fields such as opthalmology, ENT-surgery, diagnostics, wound care, micro surgery, etc.
The versatile and robust nature of the material has made it possible for it to be used as a component in various medical devices worldwide. Over the past 4 decades, we have constantly improved on the quality of the material based on experience and the knowledge acquired form it's usage in various applications.
Our core business is to adapt our sponge material to our client's requirements, of course, within the given physical and chemical properties of the sponge material. In other words, we custom manufacture our sponge material in order to meet specifications and challenges of our clients.
Absorption

Our Sugi® cellulose sponge material has an absorptive capacity ranging from 1200% - 2100% depending on the material specification.
We attribute this high absorptive capacity to the following physical properties: open pore structure, cellulose affinity to water, adhesion, cohesion and van der Waals forces Furthermore, the Sugi® sponge material will not only absorb liquids, it will hold and contain the absorbed liquid. The absorbed liquid can be released by applying pressure to the sponge material. However, due to the forces described above, a certain amount of the absorbed liquid will remain in the sponge material until it slowly evaporates.
The capability of absorbing and holding a specified liquid is crucial in certain applications.
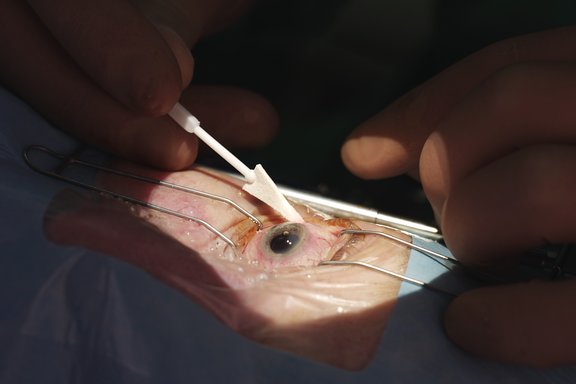
Sugi® Sponge in action
Cataract surgery
Cataract surgery consists of about 85% of all ophthalmic surgeries. Sugi® Sponge is the leading material used for this surgery.
Biocompatibility

The biocompatibility of cotton and cellulose related products has been know for several decades now. The biocompatibility of our sponge material has been certified according to ISO 10993-5, 10993-10. This cerfication releaves you of testing our material should you consider using it as a component in your device. See biocompatibility summary.
Sugi® Sponge in action
No known tissue reaction to Sugi® Sponge
With more than 45 years of experience in the field of microsurgery, ophthalmology, ENT-surgery, we can claim of a practical proven biocompatible sponge material.
Hydrophilic

Sugi® sponge material is hydrophilic with an absorptive capacity that ranges from 1200% - 2100%.
Its hydrophilic and absorptive properties are presently being effectively used in the fields of eye surgery, ENT-surgery and various microsurgical procedures. The hydrophylic property is based on open pore structure, cellulose affinity to water, adhesion, cohesion and van der Waals forces. These forces also make it possible for Sugi® to exert sufficient absorptive propertives in presence of low amounts of water molecules, e.g. in 70% isopropanol (see absorption table).

Sugi® Sponge in action
High affinity to aqueous solutions
The hydrophilic property combined with the open pore structure is the basis for excellent absorption of aqueous liquids.
Protein Binding
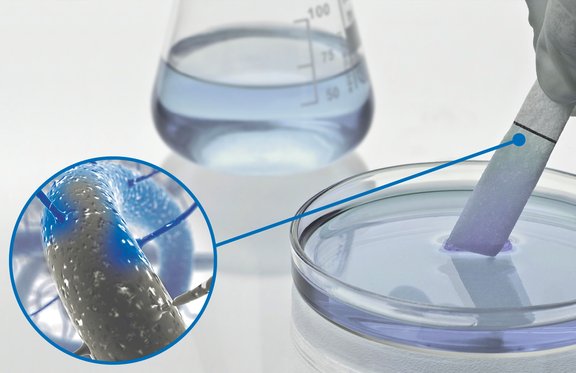
The cellulose molecules are known to bind proteins with different strengths. Our sponge material is effectively being used in the field of diagnostics in this regard.
Due to the open pore structure of our Sugi® cellulose sponge material, the surface area for binding proteins is very high. To a reasonable extent, our specialists can manipulate the area surface in order to meet certain device requirements.
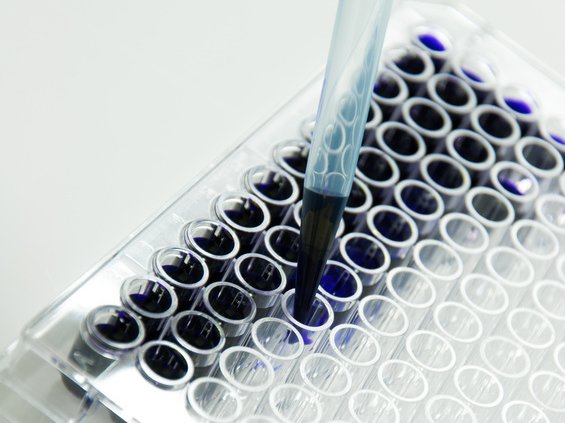
Sugi® Sponge in action
Diagnostic procedure
Many diagnostic procedures require substances and agents to bind and react in order to make certain properties visible. In this context the reaction environment is very important. Our Sugi® Sponge with its open pore structure and protein binding properties is being used as a reaction chamber for such procedures.
Open pore
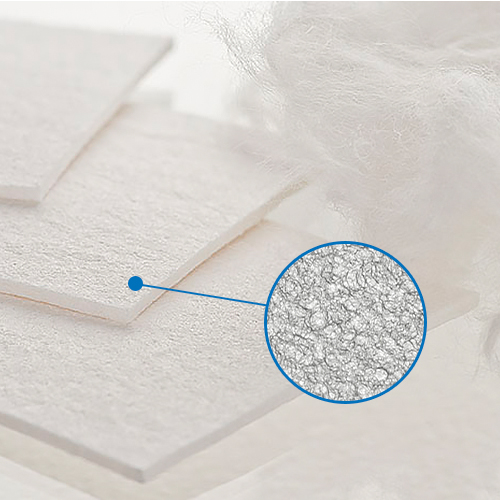
The open pore structure of the material in conjunction with its hydrophilic properties, cohesion, adhesion and the van der Waals forces secure its absorptive properties.
Furthermore, the open pore structure creates a greater surface area which on the other side creates a better environment for binding of specific agents. The cellulose fibers well embedded in the regenerated cellulose create a 3 dimensional net of pores with different pore sizes.
Sugi® Sponge material
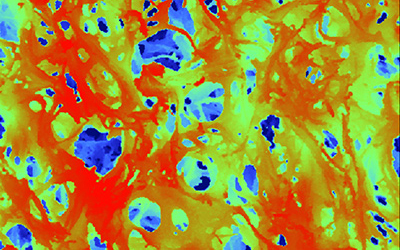
Closer view
This superficial scan of the Sugi® Sponge material displays effectively the open pore and fiber structure which are responsible for diverse properties.
Lipophobic

The lipophobic nature of the Sugi® sponge material is based to a greater deal on its hydrophilic properties. In conjunction with the hydrophilic properties, this material can be used as a barrier to fatty liquids.
Lint free

Sugi® sponge material made of pure cotton and regenerated cellulose is virtually lint-free. Due to our special manufacturing and processing procedures, the cotton fibres are tightly bond in the material. For this reason, it is the preferred cellulose material in the field of ophthalmic surgery and ENT surgery.
The tensile strength (tear strength) is an expresses the bonding of our cellulose sponge material with the regenerated cellulose (> 50 N). This value has only been achieved through experience and special manufacturing techniques.
Sterilization

The material is compatible with the following sterilization methods:
- ETO gas
- Gamma ray
Autoclave sterilization is neither recommended nor compatible due the physical properties of cellulose. The material starts degrading at a permanent temperature of approx. 110 °C.

Sterilization procedures
Sterilized products reduce significantly the risk of infection for patients. The Sugi® Sponge material is compatible with gamma ray and ETO gas sterilization procedures. For more details kindly contact our support team.
Compressible

The material is compressible and can be compressed down from 8 mm to ~0,7 mm. This can only be performed in the dry state.In the wet condition the Sugi® cellulose sponge material displays elastic properties regarding compression. The compressed material has a faster wicking rate (speed of absorption) which could become relevant for specific devices. The compression does not harm the overall absorptive capacity.
We offer assistance in determining your preferred compression ratio.
Stability

Our sponge material made of pure cotton and regenerated cellulose is stable in a variety of chemicals and solutions. Knowledge of the material stability helps determing how to use it in different devices. See stability list.
Kindly view our stability table in order to identify your substance of interest. See the Stability summary.
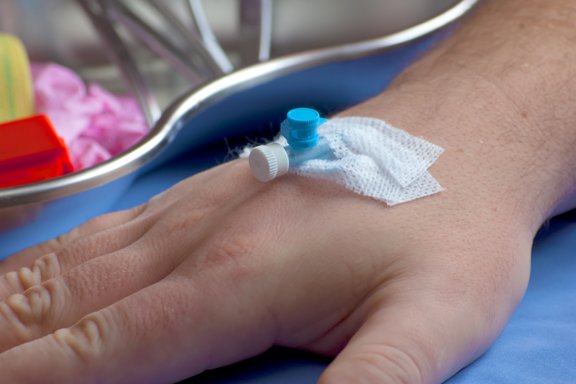
Sugi® Sponge in action
Excellent stability in alcoholic solutions
Disinfection of I.V-connectors or similar applications is very critical in the management of hospital infection. Sugi® Sponge displays high stability in concentrated alcoholic solutions. Consequently, it can be used as a reservoir for such solutions.
Tensile Strength

Due to the special manufacturing recipe Sugi® has a very high tensile strength. The tensile has to be considered as a evidence of tightly bound cotton fibres! We know of no other cellulose sponge material with equal tensile strength: > 50 N (strip 19 x 81 mm).
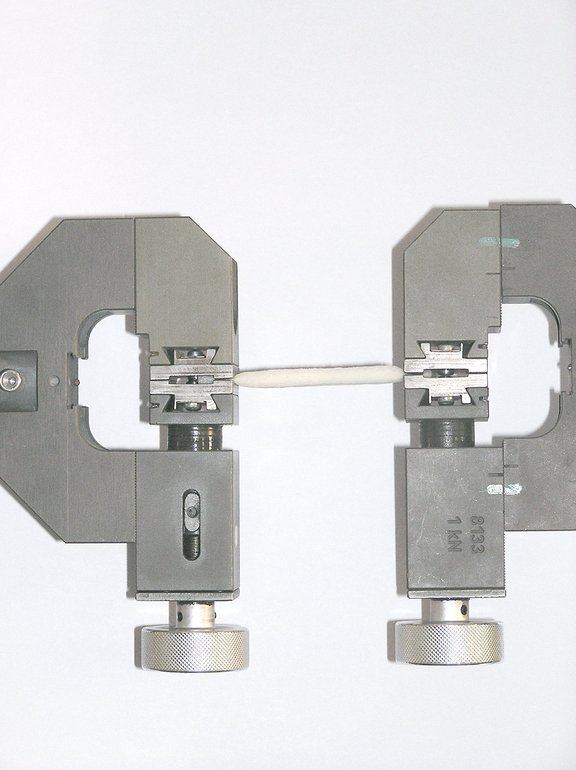
Pull strength test
The pull strength expresses the tightly bonded cellulose fibers in the Sugi® Cellulose Sponge material. Our full thickness material exhibits a pull strength of > 50 N.
Cleanliness

Sugi® is manufactured and tested according to the Ph EUR and/or DAB 10. These tests have been designed to qualify our sponge material within the auspices of a medical device manufacturer. Sugi® is not bleached. TAPPI Classification: TAPPI T213, T437, Size < 0,15 mm², Quantity/m² < 250.
Furthermore, the material is processed in a cleanroom environment of ISO Class 8 with bioburden levels of < 50 CFU/g.

